Zeniva® ZA-520 Rods
The manufacturing factily for Zeniva® PEEK in Alpharetta, GA is ISO 13485 registered and the relaevant aspects of current Good Manufacturing Practices are also applied. Our biomaterial manufacturing processes are carefully validated, and enhanced controls provide product traceability. In addition, all materials are tested in an accredited lab that is ISO 17025 compliant.
The chemical, physical and mechanical properties of finished parts are related to the processes utilized in producing the finished part, for example, molding, machining, annealing, sterilzation.
一般属性
使用 Prospector 建立免費帳戶時,可造訪 Zeniva® ZA-520 Rods 的完整資料表詳細資訊。您將找到有關物理、機械和硬度規格的完整資訊
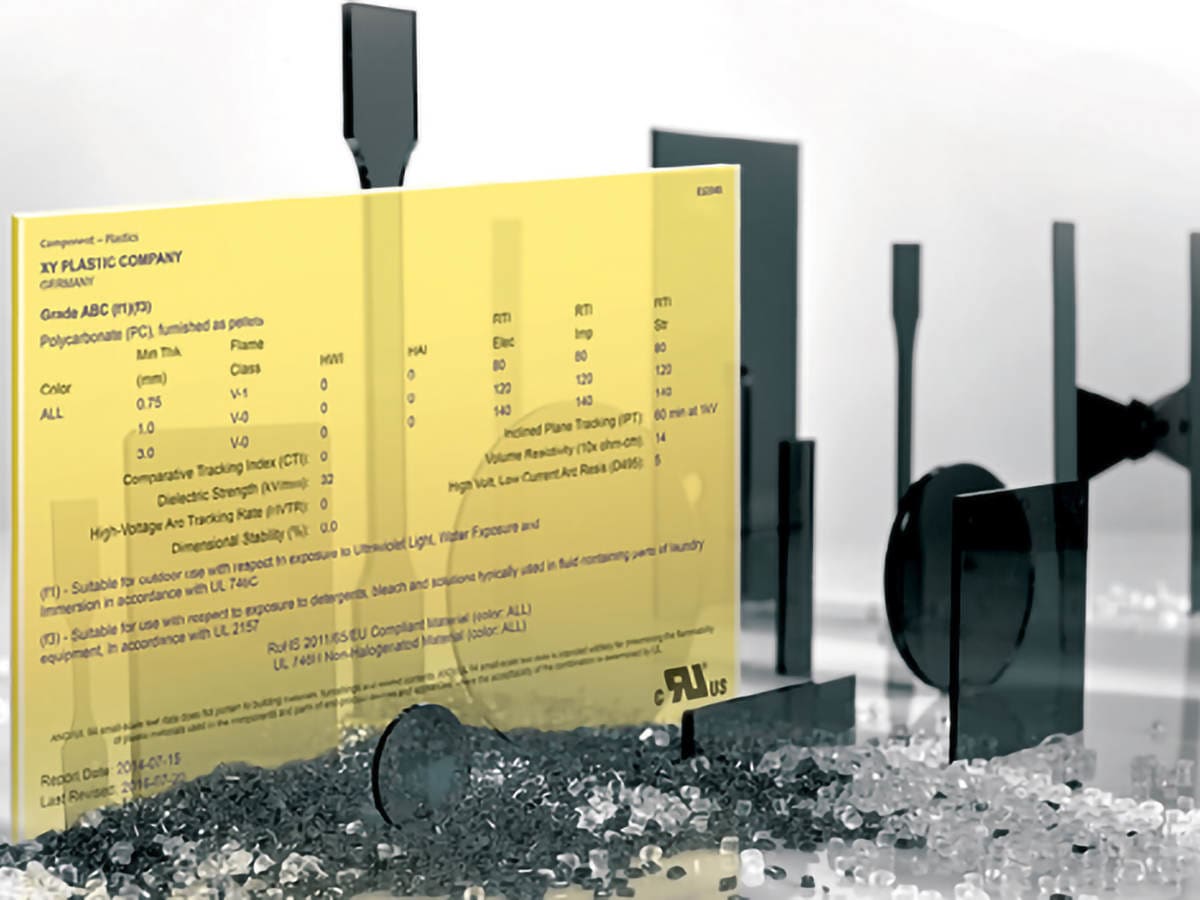
Technical Datasheet
下载文档加工
Find specific processing information for Zeniva® as well as general information for the 聚醚醚酮 generic family. 注册 或 登录 了解更多信息。