Celanex® 2401 MT
Celanex 2401MT is an unfilled, medium flow PBT grade for injection molding processing. Celanex 2401MT is a special grade developed for medical industry applications and complies with: CFR 21 (177.1660) of the Food and Drug Administration (FDA) and is listed in the Drug Master File (DMF 10047 (US) / 10033 (EU)) and the Device Master File (MAF 443 (US) / 1078 (EU)) the corresponding EU and national registry regulatory requirements biocompatibility in tests corresponding to USP 23 Class VI/ISO 10993 low residual monomers no animal products
General Properties
Access the complete datasheet details for Celanex® 2401 MT when you create your free account with Prospector. You’ll find complete information on physical, mechanical and hardness specs.
Technical Datasheet
Download Document- Isothermal Stress vs. Strain (ISO 11403)
- Secant Modulus vs. Strain (ISO 11403)
- Shear Stress vs. Shear Rate (ISO 11403)
- Viscosity vs. Shear Rate (ISO 11403)
Processing
Find specific processing information for Celanex® PBT as well as general information for the Polybutylene Terephthalate generic family. Register or Sign In for more information.
Where To Buy
You can purchase Celanex® 2401 MT from 7 distributors or manufacturers. Register or Sign In for more information.
Find UL Yellow Cards
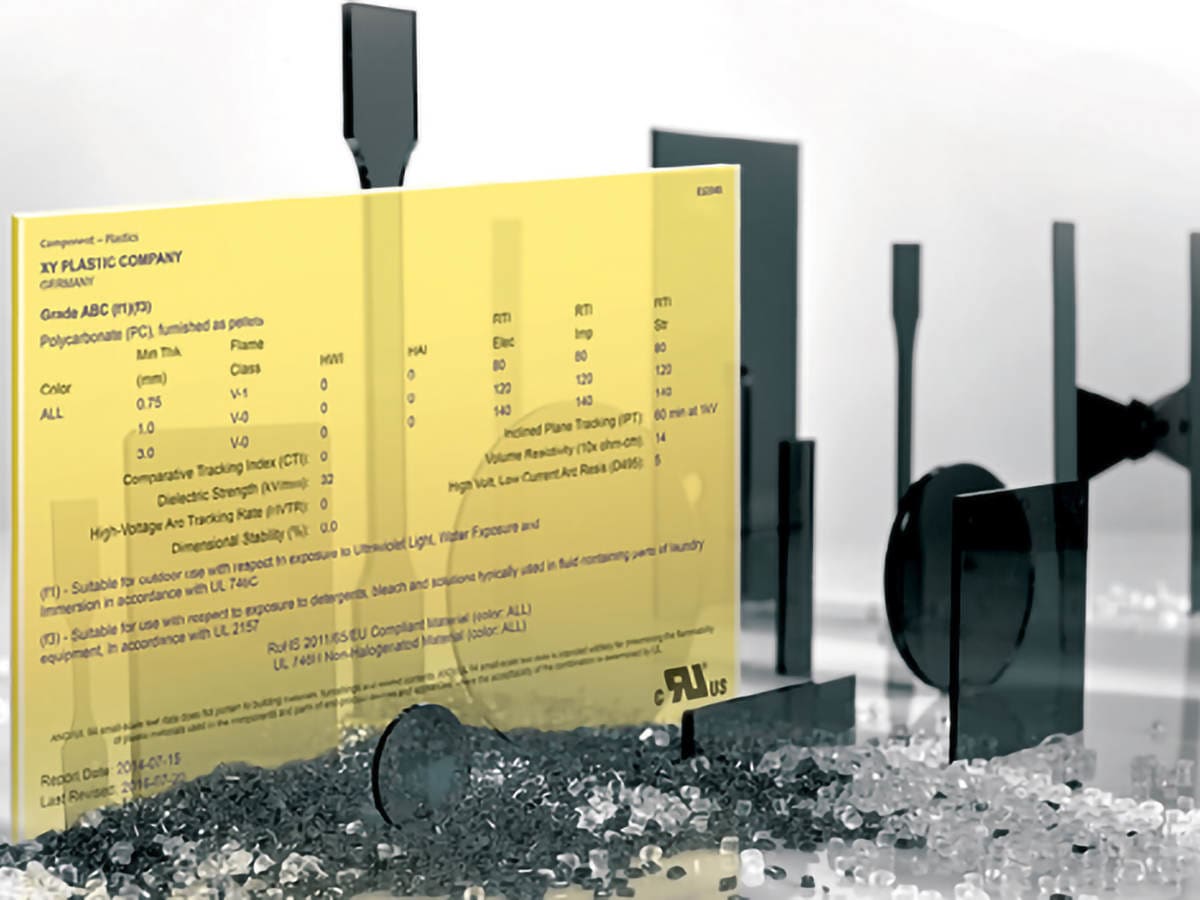
UL's Yellow Cards are a globally recognized safety and quality guarantee, a certified demonstration of how plastics have met a specific set of performance credentials.

PROSPECTOR
Find solutions, not just materials.
When you join Prospector, you leverage the dynamic tools and features to find solutions fast.
Register Today





