Optix® CA-1000 IG
CODE COMPLIANCES
OPTIX CA-1000 IG complies with FDA regulation 21 CFR 177.1010 (acrylic and modified acrylic plastics, semi-rigid and rigid) for food contact uses. OPTIX CA-1000 IG also complies with USP Class VI biocompatibility specification for use in medical applications.
APPLICATIONS
Products in contact with food, optical lenses and other parts for medical devices
General Properties
Access the complete datasheet details for Optix® CA-1000 IG when you create your free account with Prospector. You’ll find complete information on physical, mechanical and hardness specs.
Technical Datasheet (English)
Download DocumentProcessing
Find specific processing information for Optix® Acrylic Polymers as well as general information for the Acrylic, Polymethyl Methacrylate generic family. Register or Sign In for more information.
Where To Buy
You can purchase Optix® CA-1000 IG from 7 distributors or manufacturers. Register or Sign In for more information.
Find UL Yellow Cards
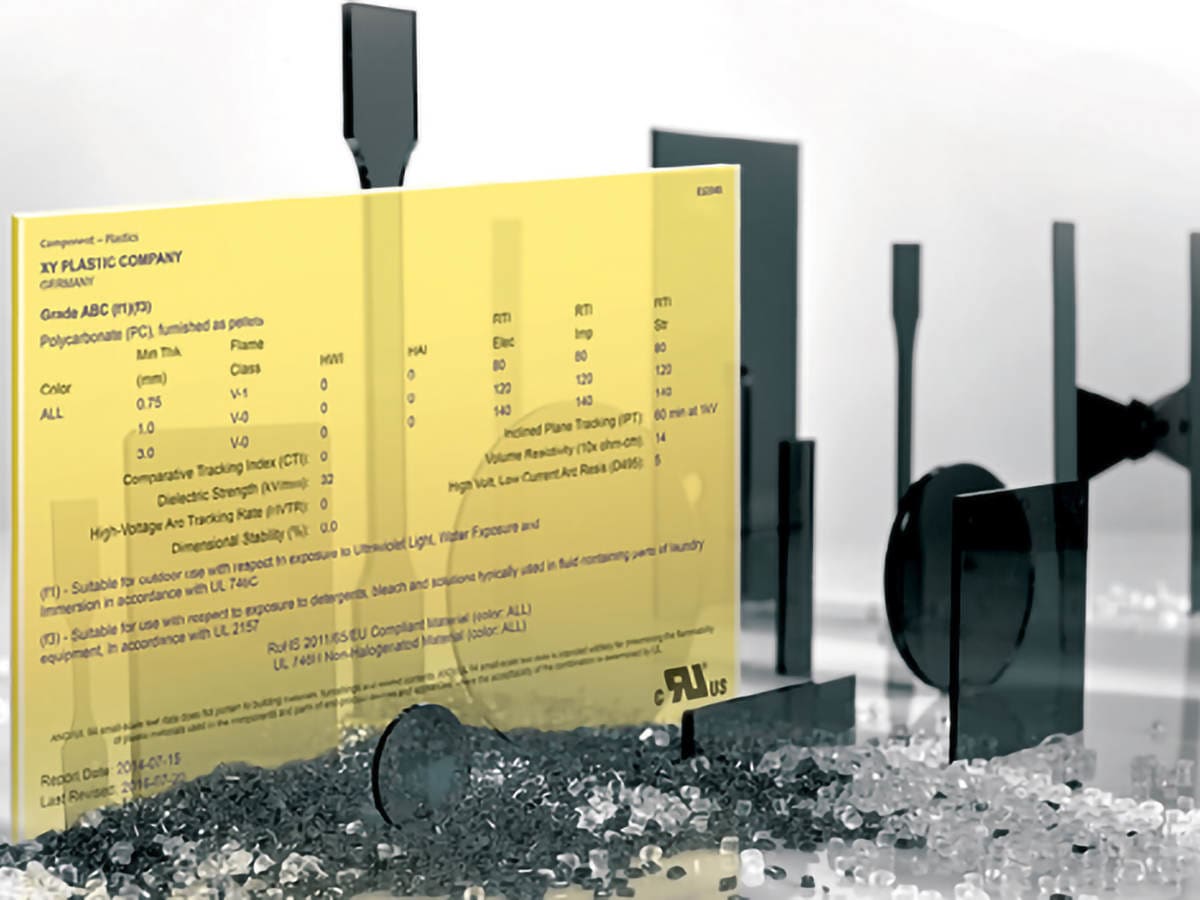
UL's Yellow Cards are a globally recognized safety and quality guarantee, a certified demonstration of how plastics have met a specific set of performance credentials.

PROSPECTOR
Find solutions, not just materials.
When you join Prospector, you leverage the dynamic tools and features to find solutions fast.
Register Today





