Zeniva® ZA-500
The manufacturing facility for Zeniva® PEEK in Alpharetta, GA is ISO 13485 registered and the relevant aspects of current Good Manufacturing Practices are also applied. Our biomaterial manufacturing processes are carefully validated, and enhanced controls provide product traceability. In addition, all materials are tested in a lab that is ISO 17025 accredited. This product meets the requirements of the current version of ASTM F 2026.
Zeniva® ZA-500 is offered as resin pellets and fabricated forms such as rods and plates. The chemical, physical, and mechanical properties of finished parts are related to the processes utilized in producing the finished part (for example, molding, machining, annealing, sterilization, etc).
An FDA MAF is available and a PMDA MF is available in Japan.
General Properties
Access the complete datasheet details for Zeniva® ZA-500 when you create your free account with Prospector. You’ll find complete information on physical, mechanical and hardness specs.
Technical Datasheet
Download DocumentProcessing
Find specific processing information for Zeniva® as well as general information for the Polyetheretherketone generic family. Register or Sign In for more information.
Where To Buy
You can purchase Zeniva® ZA-500 from 1 distributors or manufacturers. Register or Sign In for more information.
Find UL Yellow Cards
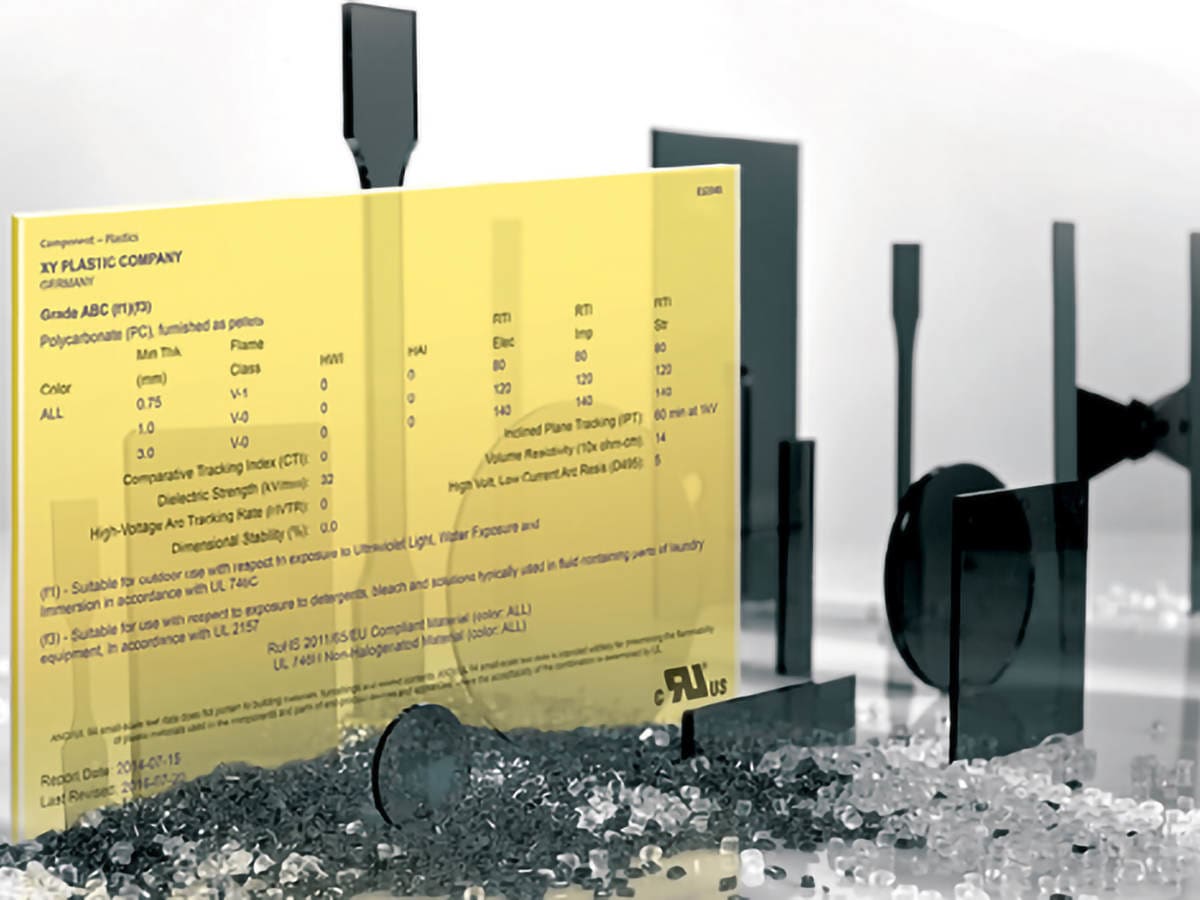
UL's Yellow Cards are a globally recognized safety and quality guarantee, a certified demonstration of how plastics have met a specific set of performance credentials.

PROSPECTOR
Find solutions, not just materials.
When you join Prospector, you leverage the dynamic tools and features to find solutions fast.
Register Today





