Tritan™ EX401
Tritan EX401 can be converted into parts using injection molding, injection stretch blow molding (ISBM), and extrusion blow molding techniques.
Tritan EX401 may be used in repeated use food contact articles under United States Food and Drug Administration (FDA) regulations. Contact Eastman representative for details on global food contact regulatory clearances.
Tritan EX401 is included in Eastman Chemical Company’s Customer Notification Procedure which details our policy for customer notification when significant changes are made in Tritan EX401 sold into the infant care market. This procedure provides the infant care industry an added layer of confidence in the consistent quality and performance of Tritan.
Key Attributes
- Chemical resistance
- Clarity
- Global food contact regulatory clearances
- Heat resistance
- Hydrolytic stability
- Impact resistance
- Processing ease
- Sterilization capable via steaming or boiling water
Applications
- Baby bottles/sippy cups
- Childcare items
- Infant/toddler
- Small appliances non-food contact
General Properties
Access the complete datasheet details for Tritan™ EX401 when you create your free account with Prospector. You’ll find complete information on physical, mechanical and hardness specs.
Processing - Injection Molding Guide (English)
Download DocumentProcessing
Find specific processing information for Tritan™ Copolyester as well as general information for the Copolyester generic family. Register or Sign In for more information.
Where To Buy
You can purchase Tritan™ EX401 from 6 distributors or manufacturers. Register or Sign In for more information.
Find UL Yellow Cards
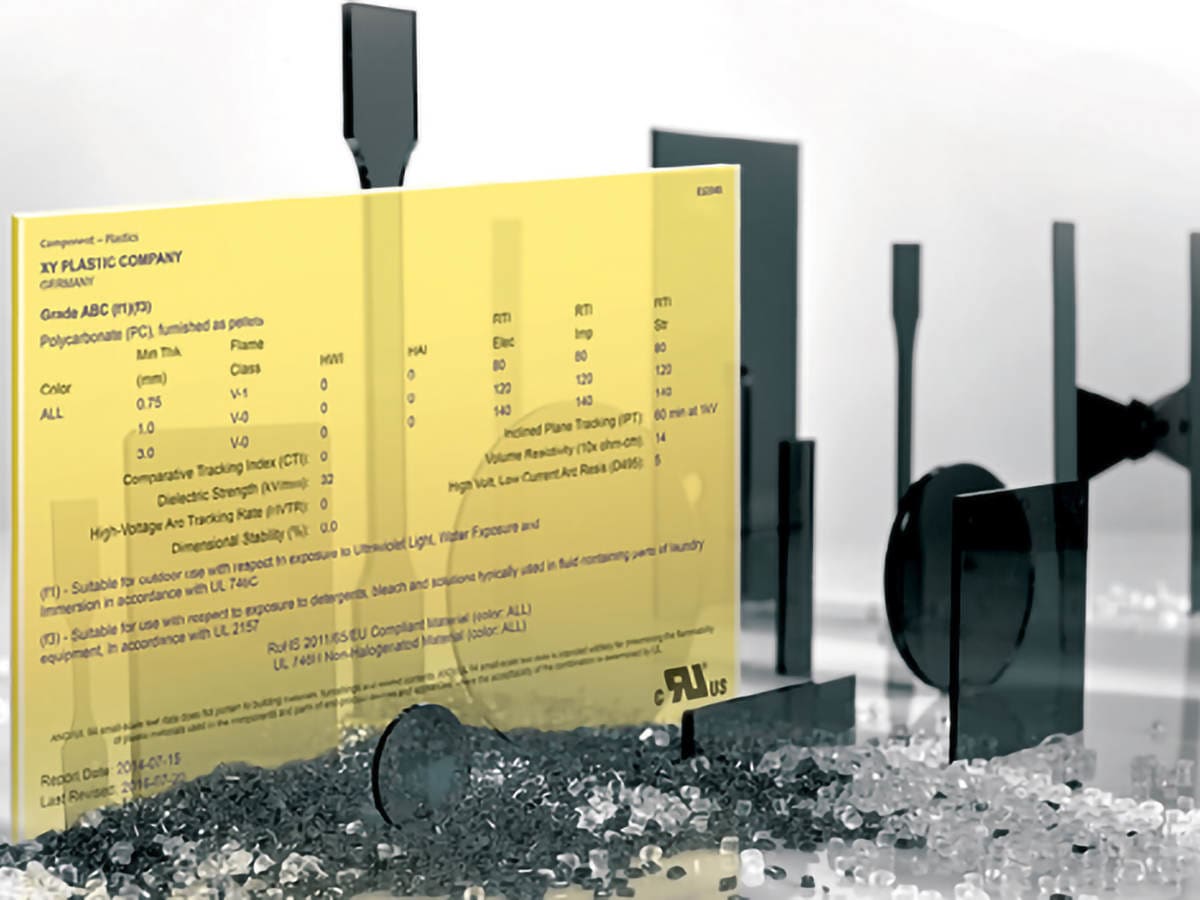
UL's Yellow Cards are a globally recognized safety and quality guarantee, a certified demonstration of how plastics have met a specific set of performance credentials.

PROSPECTOR
Find solutions, not just materials.
When you join Prospector, you leverage the dynamic tools and features to find solutions fast.
Register Today






